How To - Masses & Solutions 1 - Chemistry
Hey there, I have had a lot of trouble with this one - probably also because I didn't practice so much...
Making solutions and solvents of solutions is difficult, to me.
So today we are gonna fix that ._.
To calculate solutions with a concentration, there is a very simple formula the so called
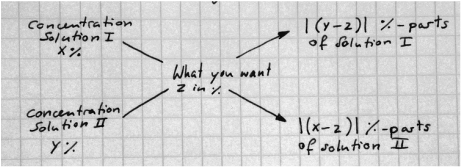
Andreas-Cross, this is what it looks like:
We have two overlapping arrows with the target solution (Sol Z) and it's concentration we want to produce in the middle.
The left side shows our base material (Solution I (Sol X), Solution II (Sol Y) and their respective concentrations) and on the right we will get how many parts of each solution we need to create Sol Z, by subtracting the percentage from Sol Z from Sol X / Sol Y and taking the modulus from that.
*BUTT* Take a good look at the picture - Sol Y tells you how many parts of Sol X you need and the other way too!!
1. Example
Sol X = 35% Acid; Sol Y = 15% Acid
We want a 22% Solution mixed from Sol X and Sol Y
Tadaa - if you got to here, congrats. We need 7 parts of Sol X and 13 parts of Sol Y - the parts total to a complete amount of 20.
If you want to get 1kg of the 22% acid, divide 1000g by the total of parts and multiply it with how many parts you need for each material
This works with water too - if you want to dilute an acid i.e., just fill in 0% for the water and start calculating :)
Making solutions and solvents of solutions is difficult, to me.
So today we are gonna fix that ._.
To calculate solutions with a concentration, there is a very simple formula the so called
Andreas-Cross, this is what it looks like:
The left side shows our base material (Solution I (Sol X), Solution II (Sol Y) and their respective concentrations) and on the right we will get how many parts of each solution we need to create Sol Z, by subtracting the percentage from Sol Z from Sol X / Sol Y and taking the modulus from that.
*BUTT* Take a good look at the picture - Sol Y tells you how many parts of Sol X you need and the other way too!!
Sol X = 35% Acid; Sol Y = 15% Acid
We want a 22% Solution mixed from Sol X and Sol Y
If you want to get 1kg of the 22% acid, divide 1000g by the total of parts and multiply it with how many parts you need for each material



Comments
Post a Comment